A simplified MyProstateScore2.0 for high-grade prostate cancer
Cancer Biomarkers (2025)
Abstract
Background: The limited diagnostic accuracy of prostate-specific antigen screening for prostate cancer (PCa) has prompted innovative solutions, such as the state-of-the-art 18-gene urine test for clinically-significant PCa (MyProstateScore2.0 (MPS2)).
Objective: We aim to develop a non-invasive biomarker test, the simplified MPS2 (sMPS2), which achieves similar state-of-the-art accuracy as MPS2 for predicting high-grade PCa but requires substantially fewer genes than the 18-gene MPS2 to improve its accessibility for routine clinical care.
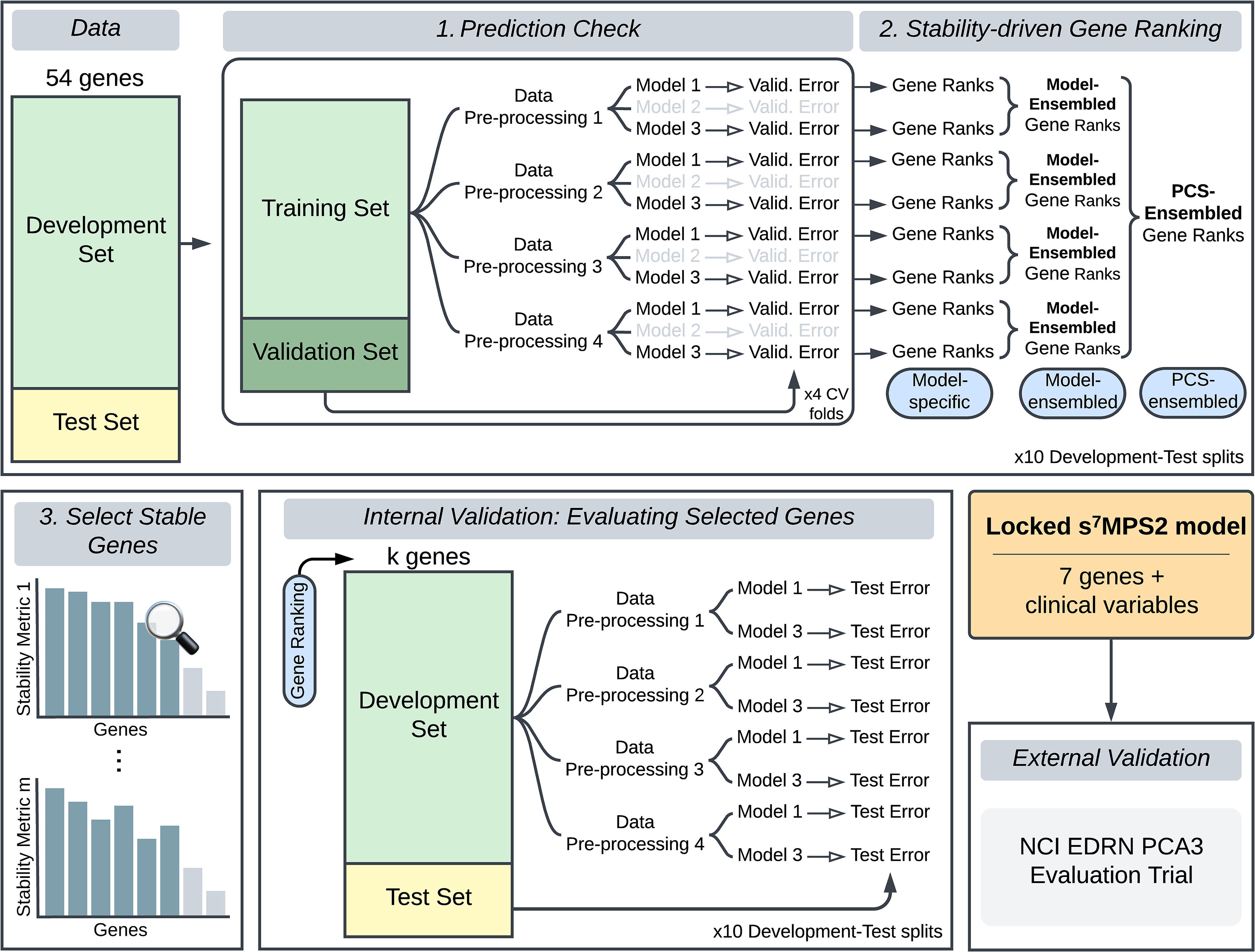
Methods: We grounded the development of sMPS2 in the Predictability, Computability, and Stability (PCS) framework for veridical data science. Under this framework, we stress-tested the development of sMPS2 across various data preprocessing and modeling choices and developed a stability-driven PCS ranking procedure for selecting the most predictive and robust genes for use in sMPS2.
Results: The final sMPS2 model consisted of 7 genes and achieved a 0.784 AUROC (95% confidence interval, 0.742–0.825) for predicting high-grade PCa on a blinded external validation cohort. This is only 2.3% lower than the 18-gene MPS2, which is similar in magnitude to the 1–2% in uncertainty induced by different data preprocessing choices.
Conclusions: The 7-gene sMPS2 provides a unique opportunity to expand the reach and adoption of non-invasive PCa screening.